Irene Hartmann, a passionate infectologist from Concordia, Entre Ríos, shared her unsettling experience about contaminated drugs that the regulatory agency ANMAT allegedly ignored. In 2023, she was taken aback when six cancer patients under her care fell ill due to a bacterial infection after receiving medication from HLB Pharma. Maria Florencia Prieto expressed her frustration saying,
“I don’t understand why they didn’t take action. Perhaps because we are from a small town in Entre Ríos.”
The incident happened nearly two years ago and endangered the lives of these breast cancer patients. This alarming revelation added to the ongoing investigation surrounding HLB Pharma and the tainted fentanyl linked to multiple deaths in La Plata’s hospital earlier this year. The situation escalated as it came to light that half of the country, along with significant healthcare entities like the Ministry of Health and PAMI, sourced medications from this controversial laboratory through bidding processes.
As the story unravels, it reveals layers of negligence and questionable practices within pharmaceutical companies like HLB Pharma and Laboratorios Ramallo. Reports highlighted that ANMAT had issued warnings regarding HLB’s manufacturing practices over time but failed to address them effectively. Moreover, HLB was on the verge of becoming a local producer for Russia’s COVID-19 vaccine Sputnik V before these scandals unfolded.
Expert insights shed light on key players like Ariel García Furfaro and Jorge Salinas connected to these pharmaceutical firms. Notably, Garcia ventured into the pharma industry by acquiring HLB around 2017 while also being associated with media acquisitions alongside prominent figures like union leader Victor Santa María. On the other hand, Salinas faced controversies related to pharmaceutical irregularities in past years.
Maria Florencia Prieto’s journey as an infectologist delves into how she uncovered a baffling medical crisis in Concordia involving contaminated dexamethasone medication administered to cancer patients every three weeks at Sanatorio Garat. She described how an unusual fever among these patients triggered an internal investigation akin to solving a Sherlock Holmes mystery.
Prieto vividly recalled moments of anxiety during their probe stating,
“Oncologists refused further chemotherapy here,”
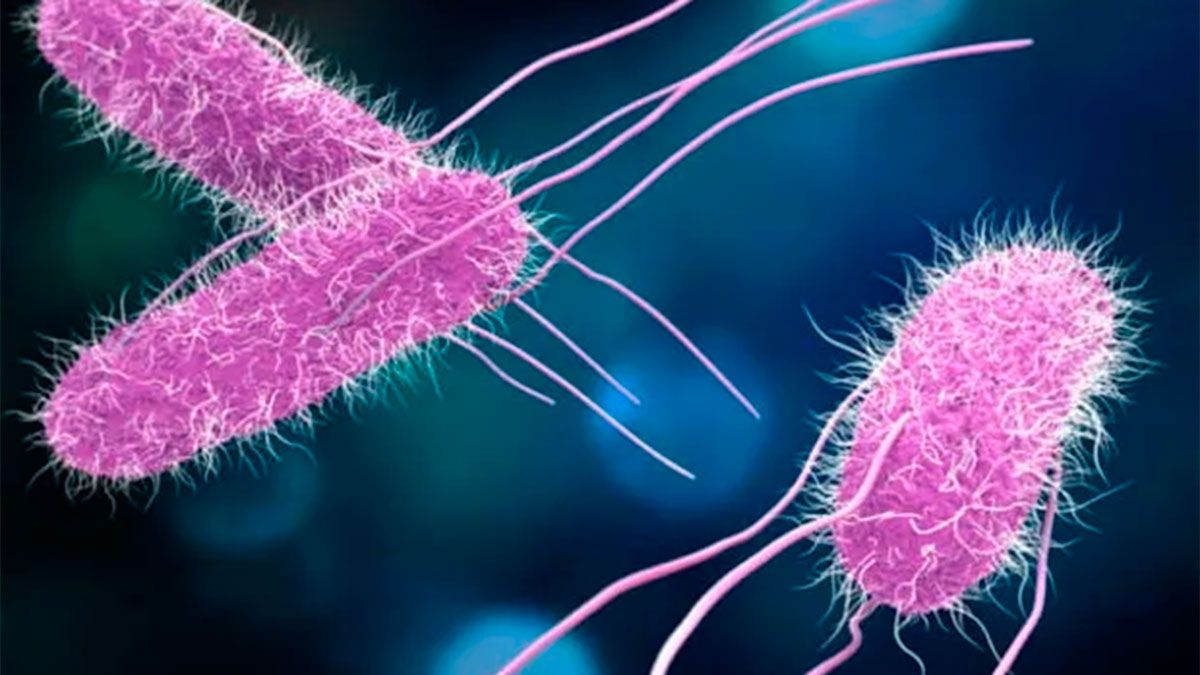
while nurses insisted on following proper protocols meticulously despite all efforts going awry due to recurrent infections caused by an unfamiliar bacterium – Ralstonia mannitolilytica discovered inside their implanted catheters.
The painstaking investigative process eventually led them to trace back contamination sources to specific batches of dexamethasone supplied by HLB Pharma where this elusive bacterium thrived within medication vials themselves – a rare occurrence that threatened patient safety significantly.
Upon discovering the contamination issue plaguing their patients’ treatments, Prieto’s team swiftly reported their findings internally before escalating it formally through appropriate channels for intervention. However, disappointment ensued when ANMAT seemingly dismissed their concerns without thorough scrutiny or follow-up actions despite concrete evidence presented by Prieto’s team regarding contaminated medications endangering patient lives.
Prieto expressed her dismay upon seeing similar incidents related to tainted products emerging from HLB later on without any substantial preventive measures taken based on previous reports ignored by regulatory authorities. Her dedication towards patient welfare remained unwavering despite facing bureaucratic hurdles hindering swift interventions needed during such critical medical emergencies.
This tale serves as a poignant reminder of challenges faced by frontline healthcare workers striving tirelessly against odds amidst systemic shortcomings requiring urgent reforms for ensuring stringent quality control measures across pharmaceutical supply chains nationwide.